- Product Details
Keywords
- 186259-63-2 buy
Quick Details
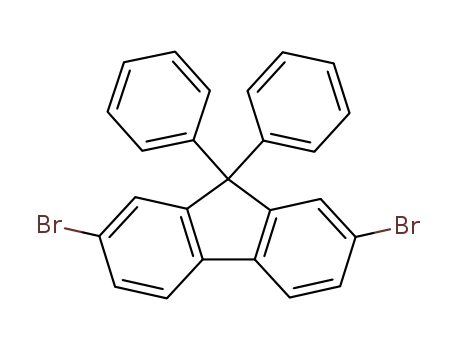
- ProName: 2,7-Dibromo-9,9-diphenylfluororene
- CasNo: 186259-63-2
- Molecular Formula: C25H16Br2
- Appearance: white powder
- Application: Pharmaceutical intermediates
- DeliveryTime: Please contact us directly to check it
- PackAge: bag; Can be packaged in accordance wit...
- ProductionCapacity: Metric Ton/Day
- Purity: 99.0%
- Storage: Store in a cool, dry place. Store in a...
- Transportation: By air or By sea or By express
- LimitNum: 10 Gram
Superiority
1.Introduction of 186259-63-2
2,7-Dibromo-9,9-diphenylfluororene has CAS registry number as 186259-63-2, and its synonyms are 2,7-dibromo-9,9
Details
1.Introduction of 186259-63-2
2,7-Dibromo-9,9-diphenylfluororene has CAS registry number as 186259-63-2, and its synonyms are 2,7-dibromo-9,9-diphenyl-fluororene; 2,7-dibromo-9,9-diphenyl-9h-fluorene; 2,7-dibromo-9,9-diphenylfluorene; 9,9-diphenyl-2,7-dibromofluorene; 2,7-dibromo-9,9-diph; 2,7-bromo-9,9-diphenyl-9hfluorene; 2,7-dibromo-9,9-diphenylfluorene,9,9-diphenyl-2,7-dibromofluorene. It belongs to product categories of fluorene derivatives, electronic chemicals, fluorene series, OLED materials, pharm chemical, electronic chemical.
2.Properties of 186259-63-2
ACD/LogP: 7.803 # of Rule of 5 Violations: 1
ACD/LogD (pH 5.5): 7.80
ACD/LogD (pH 7.4): 7.80
ACD/BCF (pH 5.5): 501781.70
ACD/BCF (pH 7.4): 501781.70
ACD/KOC (pH 5.5): 418793.80
ACD/KOC (pH 7.4): 418793.80
#Freely Rotating Bonds: 2
Index of Refraction: 1.693
Molar Refractivity: 117.97 cm3
Molar Volume: 307.501 cm3
Polarizability: 46.767 10-24cm3
Surface Tension: 55.3950004577637 dyne/cm
Density: 1.549 g/cm3
Flash Point: 311.081 °C
Enthalpy of Vaporization: 76.337 kJ/mol
Boiling Point: 520.31 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
3.Structure Descriptors of 186259-63-2
(1)InChI: InChI=1S/C25H16Br2/c26-19-11-13-21-22-14-12-20(27)16-24(22)25(23(21)15-
19,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-16H
(2)InChIKey: AJYDOCCGNIBJBY-UHFFFAOYSA-N
(3)Canonical SMILES : C1=CC=C(C=C1)C2(C3=C(C=CC(=C3)Br)C4=C2C=C(C=C4)Br)C5=CC=CC=C5